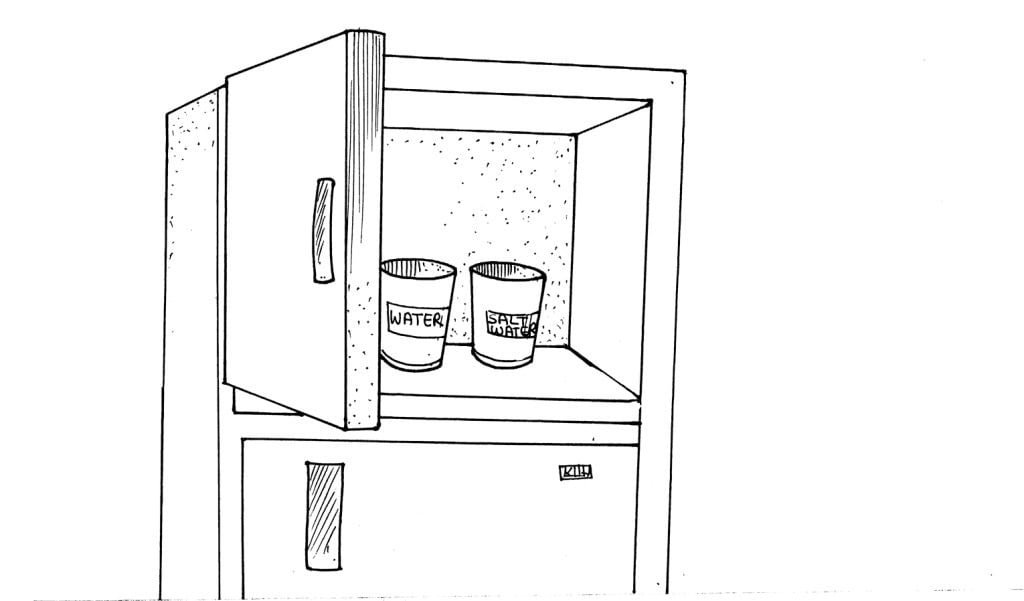
In this experiment, you will determine the effect of dissolved nutrients on the freezing rate of water. How does this affect the freezing rate of plants?
Things Required:
Salt
2 5-ounce (150 ml) paper cups
Measuring spoon-teaspoon (5 ml)
Refrigerator
Masking tape
Marking pen
Directions:
Fill both cups with water; label one salt-water and the other water. Add 1 spoon of salt to the cup labelled salt-water and stir. Place both cups in the freezer.
Observe the cups at 2-hour intervals.
This Is What Happens:
The salt water never freezes so hard as the pure water.
Science Behind It:
Salt lowers the freezing point of water. The pure water was able to freeze at a warmer temperature than the salty water. In Experiment Freezing Tissue, vegetables were observed to freeze at different rates. Their surface area affects this, but it is also possible that the amount of dissolved nutrients in the cell fluid also affects their resistance to the cold. Farmers find that bean, cucumber, eggplant, peppers, squash and tomato plants cannot stand even the lightest frost while plants like broccoli, brussel sprouts, cabbage, collards, mustard greens, radishes and turnips can withstand heavy frosts. Some of these durable plants have large leaves which support the idea that there is more to determining the freezing durability than just surface area. The materials dissolved in their leaves help to make them more frost-resistant.
