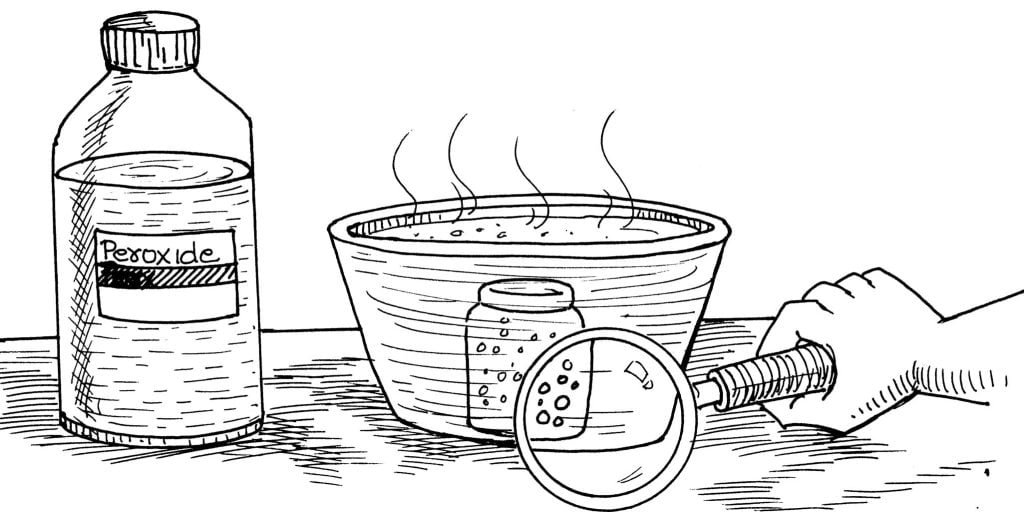
If you can add an atom, can you subtract one or release an element from a compound? Watch carefully! Oxygen will actually escape before your very own eyes in this electric and thrilling experiment.
Things Required:
Small amount of rust (scraped from old iron object)
1 tablespoonful of hydrogen peroxide
Small bottle or jar (to hold hydrogen peroxide)
Small, deep container or bowl filled with hot tap water (to submerge small bottle) modeling clay
Magnifying hand lens
Directions:
Stick a small piece of modelling clay on the bottom of the bottle. (This will anchor the bottle down and keep it steady under the water.) Put the hydrogen peroxide into the bottle and then drop in the iron rust. Lower the container into the bowl of hot water and press it against the bottom. Watch the bottle closely through the magnifying hand lens.
This Is What Happens:
Many small bubbles come from the bottle of hydrogen peroxide.
Science Behind It:
A molecule of hydrogen peroxide (H2O2) contains one more atom of oxygen than a molecule of water (H2O). When you drop the iron rust into the peroxide and place the container into the hot water, a chemical change takes place. The bubbles you see in the peroxide solution are really the groupings of those “extra” oxygen atoms being released from the hydrogen peroxide compound.
