A fine example to observe the attraction between molecules.
Things Required:
Rubber cement
1 sheet of newspaper
Scissors (These must be strong and sharp. Do not use school scissors.)
Talcum powder
Directions:
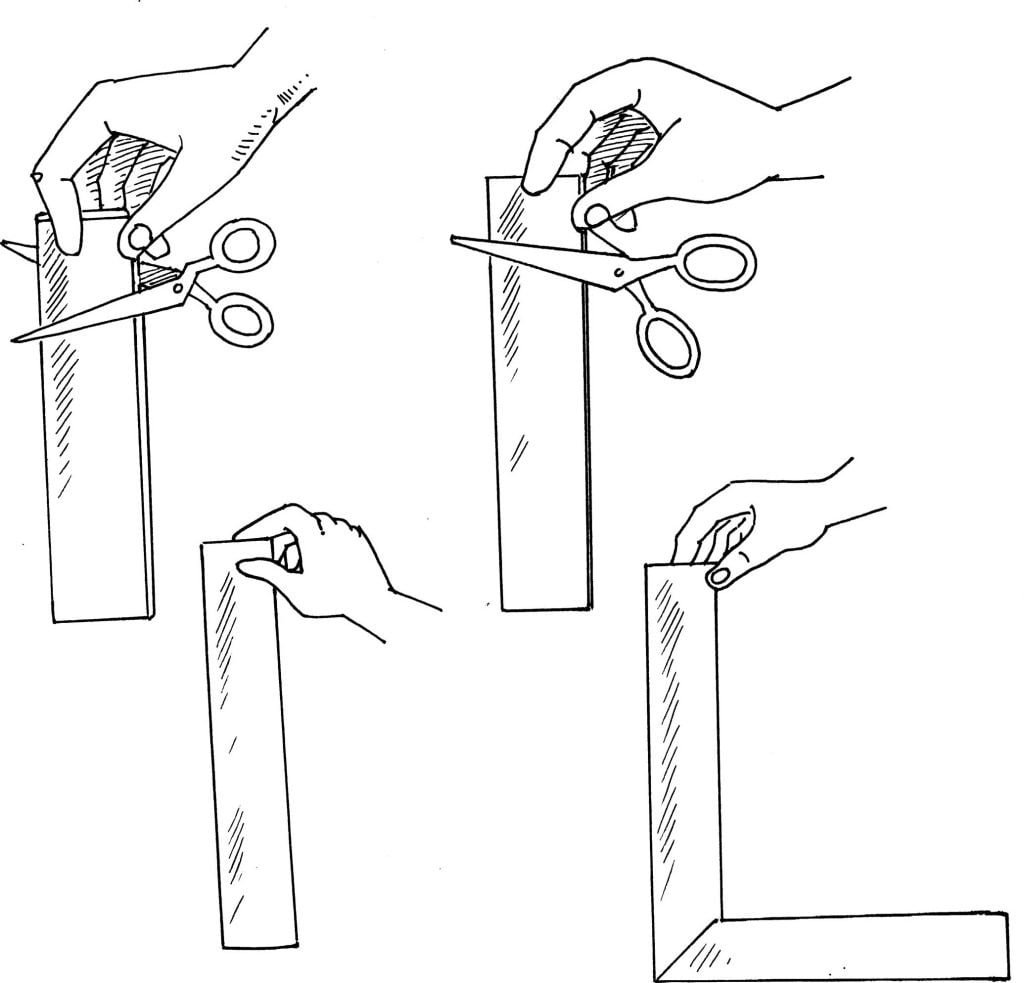
Lie the newspaper on a table. Evenly spread a thin, but solid covering of rubber cement over one-half of the newspaper page. Note: It is important not to leave spaces uncoated or globs of glue in places. Allow the rubber cement to dry for five minutes. It will feel tacky. Sprinkle talcum powder evenly over the tacky cement. Gently rub the powder to make sure that all of the cement is covered. Cut the powdered section into one-inch strips.
Hold two of the strips with the powdered sides touching. Cut across the ends of the papers. Important: Do not try to snip the paper with the ends of the scissors. Insert the paper as far into the scissors as possible, cutting with the largest part of the blade. Gently raise the end of one of the strips. Hold only the raised edge, allowing the strip to hang.
Results: Instead of two separate short strips there is one long one.
Directions:
Hold the strips with the powdered sides together again. Use the scissors to make a 45-degree diagonal cut across the ends of the paper strips. Gently raise the end of one strip. Hold only the raised edge, allowing the strip to hang.
This Is What Happens:
The paper strips are connected at a 45-degree angle to one another.
Science Behind It:
The powder is used to cover the cement so that the pieces may not stick together. The sharp edges of the scissors cut the paper. The pressure applied by the blades pushes a small amount of rubber cement along the cut surface. The molecules of the cement have a strong attraction for one another. These molecules are able to bridge the gap between the cut pieces and hold them together.
