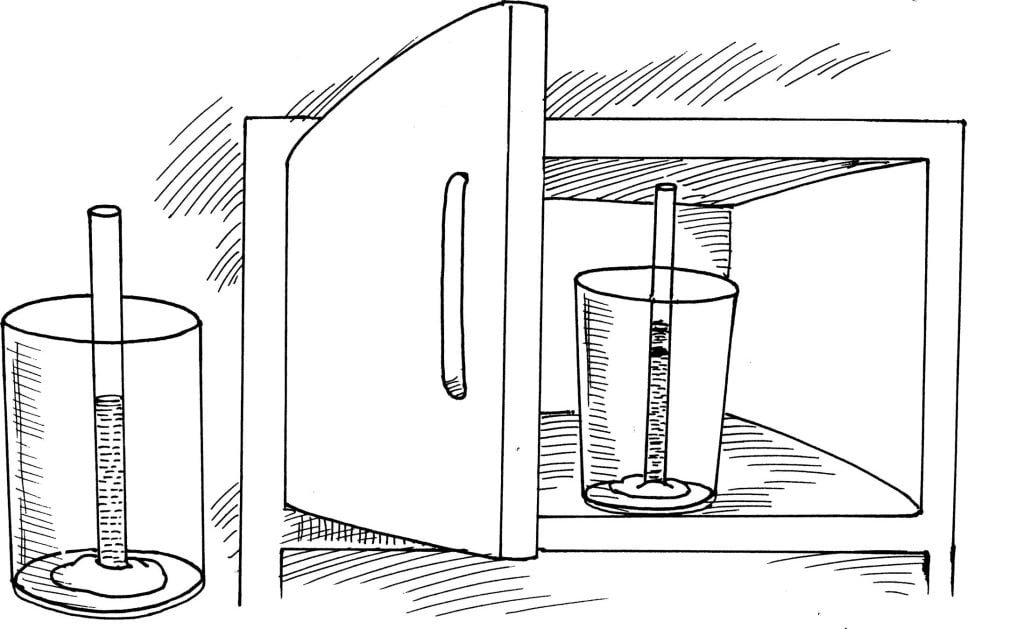
Does water expand when frozen? Find out in this easiest experiment.
Things Required:
1 straw
1 small baby food jar
Red or blue food colouring
Permanent marking pen
Clay, a piece the size of a marble
Directions:
Press the piece of clay against the inside bottom of the jar. Fill the jar with water. Add four or five drops of food colouring and stir. Slowly lower the straw into the coloured water. Push the bottom end of the straw into the clay. The straw can now stand in a vertical position. Slowly pour all of the water out of the jar. Use the pen to mark the height of the water in the straw. Place the jar in a freezer for five hours.
This Is What Happens:
The height of the frozen water is above the mark.
Science Behind It:
Water molecules are attracted to one another. When they get close enough, they bond or stick together. They do not stack together like flat boxes, but have spaces between them. Liquid water molecules occupy less volume because at the higher temperature the molecules are more flexible and can crowd together. As the temperature lowers, the molecules bond together to form a hexagonal structure. This ice structure is not very flexible and takes up more space than the same number of liquid water molecules.

