Enzymes : Enzymes are proteins molecules which facilitate specific reactions towards equilibrium.
These are commonly called organic catalysts.
Substrates : The substrates are the molecules bound to and acted on by enzymes.
Nature of enzymes : All enzymes are proteins, molecules. Most of them are composed of derivative proteins. The enzyme complex is called holoenzyme. It is composed of two parts—apoenzyme and cofactor.
Apoenzyme is the protein part of the enzyme. It is heat labile and its nature is colloidal. Heat resistant part and dialysable part of the enzyme is known as co-factor. This part has the following three groups :
(i) Prosthetic group—This group is permanently bonded to apoenzyme and of organic in nature.
(ii) Coenzymes—Coenzymes areorganic compounds, but their association with the apoenzymes is temporaty i.e. they come in contact with the apoenzyme only at the time of their catalytic action. Vitamins are the essential chemical component of many coenzymes e.g. coenzyme nicotinamide adenine dinucleotide (NAD) nicotinamide adenine dinucleotide phosphate (NADP) contains the vitamin niacin; coenzyme A contains pantothenic acid; flavin adenine dinucleotide (FAD) contains riboflavin (vitamin B2) and thiamine pyrophosphate contains thiamne (vitamin B1).
(iii) Metal ions—A large number of enzymes for their catalytic function need the presence of some specific metallic ions. The usual metal ions are Zn, Cu, Mg.
There are six major classes of enzymes—
1. Oxidoreductase Oxidation or reduction as the name indicates. Formerly these were known as oxidases and dehydrogenases.
e.g., Lactic Dehydrogenase, Cytochrome oxidase.
2. Transferases Transferase catalyze transfer of one carbon group–aldehyde or ketone. e.g., Phosphodiphosp-hatase, Glutamate pyruvate trans-minase.
3. Hydrolases These enzymes do the addition of water to a variety of bonds. It results into cleavage of sub-strate.
e.g. Lypase, Sucrase.
4. Lyases Lysases remove non hydrolytically groups from substrate. e.g. Aldolase, Decarboxylase, Fumerase.
5. Isomerases As the name indicates, iso-merases catalyze isomeric changes e.g. phospho-glucomutase.
6. Ligases or These enzymes synthetases catalyze linking together different bonds.
e.g. pyruvate carboxylase.
Chemical Nature of Enzymes
All enzymes prepared till today are proteinic in character. Proteins are defined as aminoacids and are synthesized in the cell from 20 different kinds of amino acids. Amino acids have carboxyl group (COOH) and an amino group (-NH2). During enzyme formation carboxyl group of one amino acid links with the amino group of other amino acid thus forming peptide linkage. In protein 200 to 300 peptide linkages are present. The arrangement of amino acids in protein molecule can be co-related with the arrangement of nucleolides in DNA molecules. Certain enzymes need another substance like calcium and magnesium ions whose presence in small amount is responsible for the action of a given enzyme. In such case the term co-enzyme is used. A complete enzyme, commonly known as holoenzyme, consists of co-enzymes, and a protein apoenzyme. When cell secrete enzyme, these are often first produced in an inactive form. These inactive forms of enzymes are said to be the pro-enzymes.
Mode of action
All enzymes are a kind of catalysts, therefore during biochemical reaction in the body, they first combine with the substrate to form an intermediate complex before yielding the products of the reactions. In the process, the substrate molecules are thought to fit into the active sites located on the surface of the enzyme molecule just as one particular kind of key opens one particular kind of lock. This gives rise the rapid formation named by adding the suffix as to the name of the substrate on which they act. Some of them are given below:
(i) Proteinases for the breakdown of protein
(ii) Amylases for the breakdown of starch
(iii) Lipases for the breakdown of fats
(iv) Nucleases for the breakdown of nucleic acids
Factors which affect Enzyme Activities
1. Temperature: Catalytic reaction is affected by the temperature in the same way as it affects ordinary chemical reactions. As the temperature goes up, the rate of chemical reaction also increases owing to an increase in the number of activated molecules. But when the temperature goes up above certain limit the enzyme loses its activity as they are proteins. Enzymes may be completely destroyed at high temperature. Therefore, for every enzyme under a given set of conditions there is a temperature at which the activity of enzyme is at a maximum. This is called the optimum temperature.
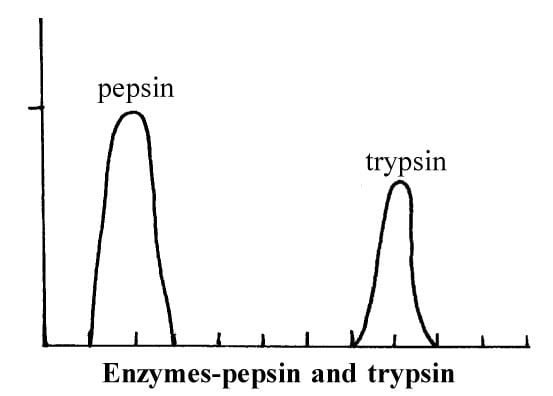
2. The pH effect : There is optimum value of pH for every enzyme at which the most rapid activity occurs. Extreme acidity or alkanity usually causes irreversible destruction of the enzyme. Pepsin which acts in the acid medium of gastric juice, has an optimum pH of 2-0.
3. Enzyme concentration : To certain extent, the concentration of enzyme is directly proportional to the chemical reaction. An increase in the enzyme concentration would also increase the rate of reaction.
4. Substrate concentration : Substrate concentration is also responsible for the rate of reaction of the enzyme. An increase in the substrate concentration will increase the chance of a substrate molecule coming in contact with an active site.
5. Product concentration : The rate of chemical reaction is reduced with the increased concentration of product.
6. Other factors : Strong light inactivates the enzymes. Ultra violet light is very effective in destroying enzyme activity. The presence of salts may affect the enzyme activity by participating with the enzyme due to its protein nature.
